The atom-connectivity matrix, denoted by ACM, has been proposed by Spialter [97-99] for the use in computer-oriented chemical nomenclature. This matrix represents the structural formula of a molecule and is given by:
[ACM]ij= |
bij if vertices i and j are adjacent |
| si if i = j (7) | |
| 0 otherwise |
where bij is the bond order between atoms i and j, and si stands for the chemical symbol of the atom i. The values of bond orders in most cases are 1, 1.5, 2, and 3 for single, aromatic, double and triple bonds, respectively. Below we give as an example the atom-connectivity matrix of 1-bromo-2-chlorocycloprop-2-ene whose structural formula is presented in Figure 9.
Figure 9. Structural formula of 1-bromo-2-chlorocycloprop-2-ene.
ACM= |
C |
1 |
1 |
1 |
0 |
0 |
1 |
||
1 |
C |
2 |
0 |
1 |
0 |
0 |
|||
1 |
2 |
C |
0 |
0 |
1 |
0 |
|||
1 |
0 |
0 |
Br |
0 |
0 |
0 |
|||
0 |
1 |
0 |
0 |
Cl |
0 |
0 |
|||
0 |
0 |
1 |
0 |
0 |
H |
0 |
|||
1 |
0 |
0 |
0 |
0 |
0 |
H |
If only the molecular skeleton without hydrogen atoms is considered, then one gets the hydrogen-suppresed structure. Spialter called the corresponding structural matrix the hydrogen-suppressed atom-connectivity matrix, denoted by HS-ACM [98,100]. The advantage of using HS-ACM instead of ACM at that time (1964) was in reducing the size of the matrix to save computer time.
the HS-ACM matrix for 1-bromo-2-chlorocycloprop-2-ene is as follows:
HS-ACM= |
C |
1 |
1 |
1 |
0 |
||
1 |
C |
2 |
0 |
1 |
|||
1 |
2 |
C |
0 |
0 |
|||
1 |
0 |
0 |
Br |
0 |
|||
0 |
1 |
0 |
0 |
Cl |
It should be pointed out that the roots of the atom-connectivity matrix concept go back at least to Baladin [101], though there are indications that this concept is even older [102]. Balandin constructed property matrices by using the symbols of atoms making up the molecule as diagonal elements and molecular properties such as the interatomic distances (in Å), bond dissociation energies (in kcal) and vibrational force constants (in cm-1 × 104) as off-diagonal elements.
The important step in the development of the concept was made by Wheland. In 1946 [103] he represented molecules as tableaux (connection tables). This Wheland contribution may be regarded as a starting point of the computer-oriented chemical documentation [104]. The Wheland tableau for 1-bromo-2-chlorocycloprop-2-ene is given below and is practically identical to the ACM matrix of Spialter.
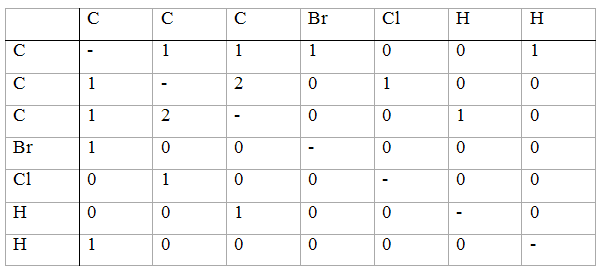
Since Wheland did not pursue his idea, this result of his remained practically unknown. Thus, for example, Spialter did not mention the Wheland tableaux in his papers. After Wheland, several authors, located either in industry or at the former National Bureau of Standards (now the National Institute for Standards and Technology) in Washington, D.C, developed methods for computer storage and retrieval of chemical structures [105-107]. Spialter himself was at Wright-Patterson Air Force Base in Fairborn near Dayton (Ohio). Apparently the need for computer-based systems for chemical documentation was perceived much earlier in applied research than in academic research.